Alim Louis Benabid
Joseph Fourier University, Grenoble

Mahlon R. DeLong
Emory University School of Medicine
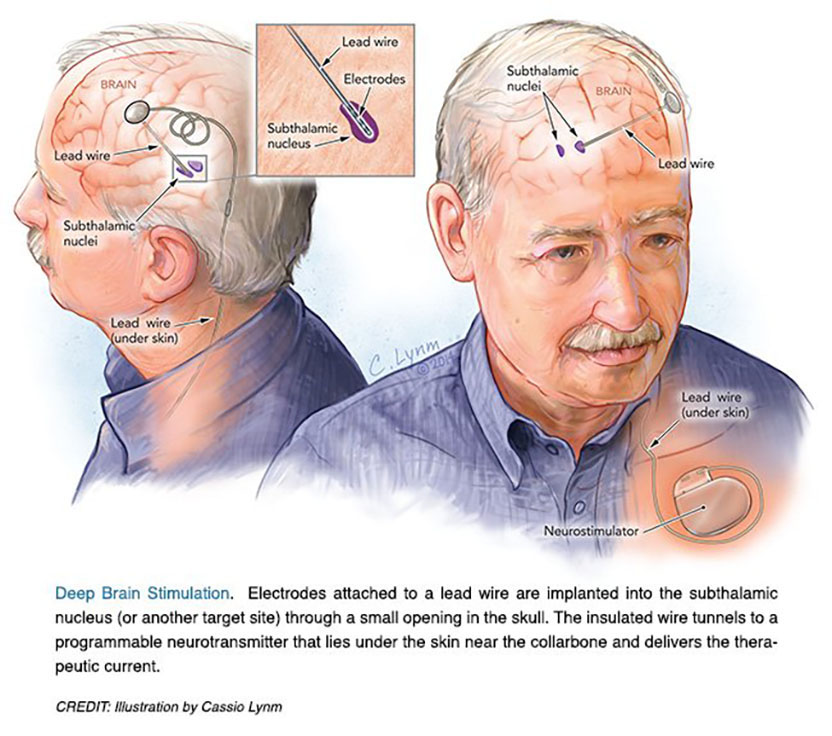
The 2014 Lasker~DeBakey Clinical Medical Research Award honors two scientists who developed deep brain stimulation of the subthalamic nucleus, a surgical technique that reduces tremors and restores motor function in patients with advanced Parkinson’s disease. Mahlon R. DeLong (Emory University School of Medicine) formulated a new model for the brain’s circuitry and exposed a fresh target for this illness. Alim Louis Benabid (Joseph Fourier University, Grenoble) devised an effective and reversible intervention that remedies neuronal misfirings. Their work has culminated in an effective treatment for more than 100,000 individuals worldwide with severe illness who suffer from complications of levodopa therapy.
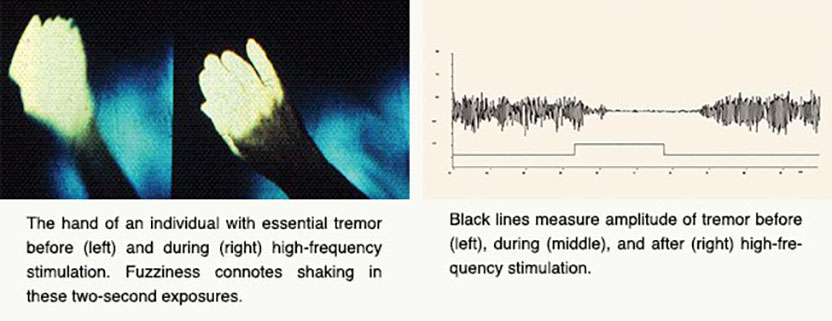
Parkinson’s disease (PD), perhaps best known for its tremor, slows and stiffens movements. From the 1940s through the 1960s, surgeons battled the ailment by destroying regions of the brain, chosen more by trial and error than by a clear understanding of neural misbehavior. The so-called lesions created by these operations often delivered spectacular and stable effects, counteracting the tremor and, to some extent, other features of PD. Even slight misplacement, however, brought complications rather than benefits. Such damage was permanent, as dead tissue could not be revived.
Award presentation by Greg Petsko
 In 1972, the novelist Michael Crichton, whose Harvard Medical School education seems primarily to have turned him into a techno-Luddite, wrote a novel called The Terminal Man. It told the story of Harry Benson, who has electrodes implanted into his brain to suppress his psychomotor epileptic seizures. The electrodes are controlled by a small computer with a power pack under the skin in his shoulder. Unfortunately, this being a Michael Crichton novel, the technology is flawed and Benson is psychotic, and — well, you can probably guess how this turns out.
In 1972, the novelist Michael Crichton, whose Harvard Medical School education seems primarily to have turned him into a techno-Luddite, wrote a novel called The Terminal Man. It told the story of Harry Benson, who has electrodes implanted into his brain to suppress his psychomotor epileptic seizures. The electrodes are controlled by a small computer with a power pack under the skin in his shoulder. Unfortunately, this being a Michael Crichton novel, the technology is flawed and Benson is psychotic, and — well, you can probably guess how this turns out.
That same year, 1972, a young medical doctor in France named Alim-Louis Benabid became a staff neurosurgeon at Joseph Fourier University in Grenoble while also beginning his studies for an eventual doctorate in physics. And also in 1972, in the United States, another young doctor, Mahlon DeLong, was finishing up his time as a research associate in the National Institute of Mental Health.
Eventually, the work of these two men would coalesce into something strikingly similar, sort of, to the device in The Terminal Man. But its use, Michael Crichton could never have dreamed up: a new therapy for Parkinson’s disease.
Acceptance remarks

Acceptance remarks, 2014 Lasker Awards Ceremony
I was born in France during World War II and raised as a teenager during the Algerian War. My brothers and I were told by my father and my mother that peace was the highest value to pursue. My father, who was an MD, wanted me to be a physician and my mother who was a nurse wanted me to be a physicist. To preserve peace, I had no other choice than being both.
I would say, then, that my life has been a science fiction novel, my story being based on the patient’s problems and expectations, the use of increasingly sophisticated tools in the battles, the thrill of facing challenges, and the delight of solving them, with sometimes happy ends for the patients, and sometimes not.
Every chapter has been an adventure shared with mentors, friends, students. The tempo was made by the goals to reach, the deadlines to meet, and the odds to overcome. Everyone needs to know how much worthwhile are his own achievements. The first and most important reward comes from the patients. Recognition and prizes are the milestones of a career. Some, such as the Lasker~DeBakey Award, are unreachable horizons you dare to dream of, without really believing the dream could become true.
Being considered for the Lasker~DeBakey Award is itself an honor. Being actually awarded is an incredible shock, and the honor this award conveys is even greater when you share it with a scientist and a friend that you respect and admire, such as Mahlon DeLong. I thank you all for your trust and belief and acknowledgement in what I tried to achieve.

Acceptance remarks, 2014 Lasker Awards Ceremony
I don’t recall when I realized I wanted to do research, but I have always enjoyed understanding how things work. A growing fascination with how the brain controls behavior led me to medicine and then to neurology. This took a clear direction when I found a choice research position at the NIH in the laboratory of renowned researcher Edward Evarts. Because the other obvious brain regions were already assigned to other fellows, I was asked to work on the basal ganglia, a cluster of poorly understood brain structures, and to determine their role in the control of bodily movements.
For years I never thought about finding a cure for anything but was fascinated by the progress in understanding the anatomical relations and functions of these mysterious structures. Almost two decades later, a new animal model of Parkinson’s was developed, made possible by the discovery of a toxin that produced clinical features and pathology closely resembling Parkinson’s, resulting from a loss of dopamine in the basal ganglia. Our basic research provided a solid foundation for what was to come. We found abnormal neuronal activity throughout the motor circuit of the basal ganglia and ‘struck gold’ by making a lesion in the subthalamic nucleus, which, by interrupting the circuit, almost immediately restored movement and eliminated tremor and rigidity. Most importantly, the findings provided insight into the neuronal dysfunction responsible for Parkinson’s and a strong rational for surgical intervention as well as a novel target.
Having spent much of my early career in both basic research and clinical work, I have been fortunate to see how the basic science contributes to patient care. I am also aware of how far this field, that we now call neuromodulation, has progressed.
Lasker Awards honor individuals who contribute critically to a research problem — but they also highlight the larger progress in a given field, in our case, using targeted electrical stimulation to restore function in the disrupted neural networks responsible for both neurologic and psychiatric disorders.
For nearly a century, neurologists learned about brain function by correlating the behavioral changes caused by strokes with the damage to specific regions of the brain. With deep brain stimulation, we are now not only able to treat disorders such as Parkinson’s — we’re also able to learn about brain networks and their function by observing the effects of targeted stimulation and activation. Whereas we previously thought about brain ‘centers’, we now focus on specific brain circuits and how they ‘go wrong’ in neurologic and psychiatric disorders. Understanding how neural circuits are disrupted by disease and how lesions and electrical stimulation can improve function is still poorly understood. Understanding brain function and its disorders is the last and most challenging frontier of medicine.
Some of our real progress and ‘breakthroughs’, are made, I believe, serendipitously, while we are doing other things — like just trying to understand how things work. I sometimes wonder whether, in our urgency to find cures, we sometimes omit the basic science necessary to understand how things work — and by doing so, may miss the chance of finding a new treatment.
Over the last 25 years, I mutated not only mouse genes, but changed myself, morphing from a molecular biochemist into a mouse geneticist into an electrophysiologist into a behavioral neuroscientist. These changes gave me the pleasure of always learning something new about how synaptic vesicles and synapses work, although these changes unfortunately have not improved my looks.
The 1990ies were an amazingly thrilling time! During this time, we together with our long-time collaborators Reinhard Jahn and Jose Rizo, and in parallel with Richard Scheller and others, discovered the role of SNARE and SM proteins in synaptic vesicle fusion. We identified synaptotagmins as calcium-sensors in neurotransmitter release, showed how synapses are organized by active zone proteins, and described the first synaptic cell-adhesion molecules that guide synapse formation. These discoveries and conceptual advances were the beginning of an understanding of synapses, which has now become generally recognized. During the course of our work, the studies of Jim Rothman, who received a Lasker Award in 2002 for his work on the cell biology of vesicle fusion, expanded our thinking and injected great enthusiasm into the rapidly growing field of neurotransmitter release.
I thank the Lasker Foundation — I thank you all for giving me this award, and I hope to live up to it!
Interview with Alim Louis Benabid and Mahlon R. DeLong
Video Credit: Susan Hadary